There are fists all over your body, hitting you wherever you are.
India’s stock market is caste-based, and people are forced to eat by releasing water.
Today, Master He won’t talk about China, but about our old neighbor, India.
As the first stop of China’s mobile phone going to sea, we all know that domestic brands have set up branches in India, and a friend of CSI sells a domestic brand mobile phone in India.

Mobile phone brands competing in India Image source: CBN
Looking at the shocking epidemic cases in India in his circle of friends every day, CSI asked curiously, can business still be done in this environment now?
My friend sighed, because it is impossible to open up new channels because of the epidemic. The biggest problem of existing channel sales is that logistics is not smooth, and it is really difficult to complete KPI.
Csi quipped: Looking at this posture, it is estimated that there is no chance of promotion and salary increase this year.
However, my buddy seems to be able to shrug off, saying that he lost the mulberry in the east corner and took advantage of the epidemic to make some money by investing in the Indian stock market, which is also a subsidy for his family.
Csi was surprised that the Indian epidemic was so severe that the stock market could still rise.
After a curious inquiry, Lao He found that what he said was true. Both India’s SENSEX index (equivalent to the Shanghai and Shenzhen 300) and India’s NIFTY50 index (equivalent to the Shanghai Stock Exchange 50) not only hit new highs, but also doubled compared with the epidemic low.
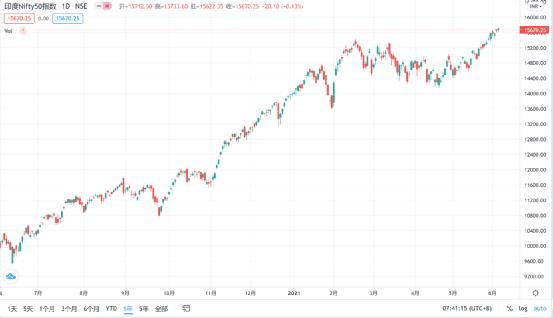
Today, CSI: Why is the Indian stock market so hot?
01
Water release is fiercer than in the United States, and Indian banks dance.
In the face of the fact that India’s stock market has hit record highs, CSI is still full of doubts. The epidemic has hit all walks of life hard. Not to mention the initial outbreak of the epidemic last year, until the recent paralysis of India’s textile industry, there are frequent reports that the Indian epidemic has affected the global shipping market. The current situation in India is in stark contrast to China’s rapid resumption of work and production.

Image source: Oriental IC
Combined with the global experience, none of the most favorable stocks, vaccine research and development, home office, and Internet giant India are touched. As for the manufacturers of gloves, masks, ventilators and other protective products, if India can produce enough of them, why do you need a dead duck to shut up and say that it is a commercial act while holding China’s aid?
What is more serious is that the number of confirmed cases in India is still as high as 100,000 every day, with 160,000 deaths in the past two months. In the past year, 100 million Indians lost their jobs, and in April this year alone, more than 7.3 million people in India lost their jobs.
Some readers here will be curious, who is the biggest star stock in Indian stock market? The answer is banking stocks.

At the beginning of the global outbreak on March 23 last year, the Indian stock index once fell by more than 10%, and the Bank of India immediately couldn’t sit still.
In just one week, the Bank of India cut interest rates by 75 basis points to 4.4%, and all banks lowered their RRR by 100 basis points to 3%. At the same time, it carried out a combined boxing rescue measure of 1 trillion rupees of QE.
India’s water release this time is not fierce. The amount of water released at a time is as high as 3.2% of GDP. Compared with Biden’s 1.9 trillion infrastructure plan, although the total amount accounts for 10% of GDP in that year, the plan will be completed in eight years, and it will cost 1.25% of GDP every year. What’s more, it is hard to say whether it can actually be spent that much.
India completely ignored these, and completed the great cause of catching up with the beauty of the Premier League in one day.

Compared with the intuitive performance that interest rate reduction means that the loan interest rate drops to reduce the burden on enterprises, RRR reduction may be a relatively unfamiliar vocabulary for many readers. Here is a simple science popularization.
The "quasi" in the RRR cut refers to the deposit reserve, which represents the proportion that banks need to deposit in the central bank to absorb deposits. The higher the reserve ratio, the less money banks can lend.
Therefore, the RRR cut means that banks can lend more, which is a policy tool to encourage banks to lend to support the economy.
Generally speaking, the RRR cut is a great positive for the stock market, and it is also true for the Indian stock market.
Compared with China’s deposit reserve ratio of 12.5% and India’s deposit reserve ratio of 3%, it is 76% lower. As you can imagine, Indian banks will be more open than China banks if they want to lend. As for whether they can really lend or not, investors don’t care about these things at present.
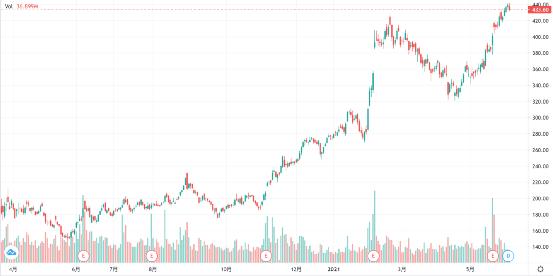
Driven by favorable conditions, Indian bank stocks began their respective rising shows.
State bank of india SBI, the largest in India, is about equal to the Industrial and Commercial Bank of India. Since the low point of the epidemic, it has increased by nearly three times, and the increase so far this year is as high as 55%. It can be said that elephants can dance.
As the second largest bank in India, ICICI, an Indian industrial credit investment bank, is mainly responsible for lending to the private sector because of its private enterprise nature. Although the private enterprises in India are bleak, it does not prevent its share price from doubling. This year, ICICI also rose by 55% compared with state bank of india and Qi Fei.
02
High-caste stocks are full, and low-caste stocks are like grass.
So are there any star stocks besides bank stocks in India?
Of course, both Tata Motors, Indian oil and gas company and Bajaj Finserv, India’s largest loan supermarket, were enthusiastically sought after by investors during the epidemic, and they also had a unified name-large-cap stocks.

Here is about the composition of Indian constituent stocks.
Among the constituent stocks of Indian stock market, banking and financial services account for the largest weight, accounting for 35.03%; Followed by energy (13.04%), IT(11.6%), consumer goods (11.17%) and automobile industry (11.02%). The weights of industries such as medicine, metals and telecommunications are all below 5%.
Is this structure very inconsistent? India’s pillar industries are not generic drugs, IT and consumer products, but banking and financial services.
Csi suddenly realized that it is no wonder that India has not set up a complete industrial system so far. It used to learn that American industry made a mistake and finance prospered the country.
Therefore, even if the operating rate of Indian pharmaceutical manufacturers is only less than 30%, India’s clothing exports have dropped by 20%. In the case that banking stocks account for one-third of the weight, as long as they keep rising, the life and death of the remaining 5,000 small and medium-sized listed companies in India’s stock market will be unknown.
So why are Indian investors so enthusiastic about large-cap stocks? The answer lies in the institutional setting of Indian stock market.
Although India implements the registration system, the Indian Securities Regulatory Commission will not restrict the companies to be listed, but India has a magical operation. If you want to go public, you need to go to the officially designated rating company for stock rating and disclosure before you can go public.

National Stock Exchange of India
At first glance, there seems to be nothing wrong with it. China also needs to be rated when issuing bonds.
But if you think about it carefully, doesn’t this so-called designated agency represent the official opinion? In other words, when a company goes public, the official gives you a rating. If it can get 3A, it will naturally be sought after. If the rating is average, I’m afraid no one cares.
If you want to stand out among thousands of listed companies in India, if you don’t get a good rating, you will basically die at the starting point.
To put it bluntly, even the stock market in India is a "caste system"! Whether the stock is good or not is decided on the day of birth!
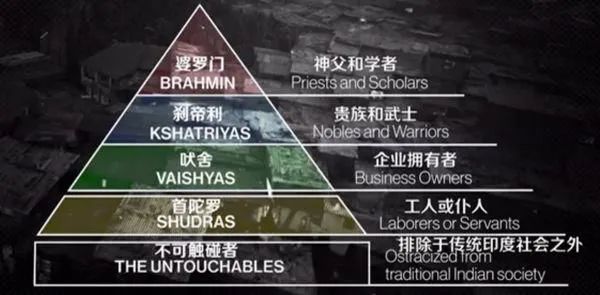
India-100 million people and 1.3 billion "cattle"
("Cattle" is a sympathetic expression of netizens.
Used to describe the actual living conditions of the people at the bottom of India)
However, these component companies have a great career, and their ratings will naturally not be worse. Newly listed companies are basically difficult to compete with them, and they will only divert companies with the same poor ratings. These companies with average qualifications can only accelerate the involution, resulting in their stock performance being even worse, and no one cares. Investors look at the investment in small-cap stocks and naturally switch to component stocks, forming a situation in which the strong will remain strong.
03
Predators cut leeks and run away, while retail investors are basically dead.
So the Indian stock market is hot, have the people got a piece of it? The answer is no!
According to the data released by the Indian Stock Exchange in 2018, there are only over 2 million Indian investors, and the latest investor data predicted by American investment banks is less than 3 million, even less than three thousandths of the population.
3 million! Maybe there are not as many investors as there are in Shenzhen. It’s ridiculous, okay!
In fact, it is not difficult to understand that 90% of India’s transactions are in cash, and it is not surprising that 99.7% of Indians are not shareholders.

Indian people queuing for money Image source: People’s Daily
Therefore, Indians who can afford to speculate in stocks are basically so-called people, not big capitalists or executives under capitalists.
Instead of doing business well, it is better to take advantage of the epidemic to sell badly, let the government throw money, speculate on the stock price, and make money. Private jets run for refuge in the beautiful world of Britain and the United States, which cares about the lives of ordinary people.
Compared with the stock market earning 250,000 yuan for a plane ticket, the price of 900,000 yuan for a charter flight is not worth mentioning for the rich in India. For example, Adal Bonavara, CEO of India Serum Research Institute, India’s largest vaccine manufacturer, fled directly by private jet.

Adal Ponavara
The ordinary people in India, even the poor stock market leek, are not qualified to be, and they are directly ignored by capitalists and left to fend for themselves.
Since the capitalists are rich and heartless, what is the Indian government doing?
As one of Trump’s best friends in the international community, the Bharatiya Janata Party (BJP) led by Indian Prime Minister Mordech pretends to represent the interests of the people. From its name, it can be seen that it is in sharp contrast to the Indian National Congress Party, which represents the elite of the Indian establishment.
But in fact, Modi, as a political veteran, is no different from his colleagues in the Congress Party in kneeling on capitalists.
Compared with Trump, at least he really built the border wall. Modi is a complete hypocrite.

After rejecting the RECP agreement, Modi launched a 2 trillion fiscal stimulus plan in response to the epidemic, trying to promote economic recovery by supporting large enterprises to become bigger and stronger.
Boy, the epidemic situation is like this. I don’t want to save ordinary people, but I also subsidize big enterprises.
It is no wonder that the share prices of those constituent stocks have been rising like chicken blood this year. After all, they are the key targets of government support!
04
tag
It can be seen that India is worthy of being a pro-son of Europe and America with national characteristics.
On the one hand, the stock market is rising every day, the market value is soaring, and the small stocks are bleak. I have learned enough about the endogenous logic of financial capital.
On the other hand, India has carried forward its own caste system tradition and directly put a stamp on the stock butt. There is nothing we can do about it, because the gap between the rich and the poor in India is so great that even leeks are barren, so we can only rely on involution to "cut in the middle".
Although India is now enjoying a boom, the Indian government’s fiscal deficit has reached 9.3% of GDP, and the epidemic situation in India has not been effectively controlled. The high valuation brought by the economic recovery advocated by the stock market is only the entertainment of 0.2% people. However, if India continues like this, how long can the myth of the stock market last?
Source | Where does Tiequan strike (ID: tiequanhe)
Original title: "Indian stock market takes off, Indian investors lie flat"
Read the original text





























